ENDOTHELIAL PROGENITOR CELLS
| Stránky: | Moodle Lékařské fakulty Univerzity Karlovy v Hradci Králové |
| Kurz: | ELC: ENDOTHELIAL PROGENITOR CELLS |
| Kniha: | ENDOTHELIAL PROGENITOR CELLS |
| Vytiskl(a): | Nepřihlášený host |
| Datum: | čtvrtek, 3. dubna 2025, 11.07 |
Popis
Description of endothelial cells, their development and their progenitor cells incl. methods of isolation and usage in stem cell therapy.
1. ENDOTHELIAL CELLS
Endothelial cells line blood and lymphatic vessels. Endothelial cells prevent blood clotting; their surface is charged negatively due to 10 nm glycosaminoglycan layer (that repels thrombocytes). Endothelial cells are flattened cells sending thin lamellar protrusions through which gas exchange occurs – cell size is 30 x 12 x 0.3 μm. Endothelial cells are usually classified as single squamous epithelium. Nevertheless cell shape can differ in some organs – in venous sinusoids of the spleen they have characteristics of spindle-shaped cells; in postcapillary venules of the lymph node they are described as high cuboidal endothelial cells. Due to the enormous length of blood vessels – more than 96,000 km in adult huma body oorganismu člověka – the total number of endothelial cells in the human body can reach numbers 10-60 billions cells.
In addition to transport of oxygen and nutrients toward the tissues, endothelial cells play lots of other functions. In embryonic development, before blood vessels are formed, a close contact between endothelial cells and developing cells of the liver or pancreas primordia is very important for tissue growth. Endothelial cells represent one of the elements of the stem cell niche – by producing specific factors (e.g., BDNF) they control stem cell behaviour.
MARKERS OF ENDOTHELIAL CELLS
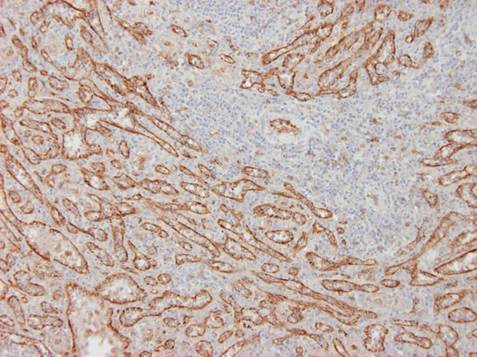
Typical markers of endothelial cells are: von Willebrand factor (Fig. 1A), CD34 (type 2), VE (vascular endothelial) cadherin, Tie2, KDR (VEGFR-2). However, there exists a phenotypic overlap between monocytes and mature vascular endothelial cells. Some markers used in endothelial detection, e.g. CD31 (PECAM), CD105 (endoglin) can be expressed by other cell types, e.g. monocytes/macrophages, and vice versa markers of macrophages, e.g. CD68 can be sometimes expressed by endothelial cells. For that reason none of the above mentioned markers is specific for endothelium only. Membranous glycoproteins can be distinguished with immunohistochemistry; polysaccharide components of endothelial cell glycocalyx can be detected with lectin histochemistry, e.g. Ulex europaeus I agglutinin (UEA-1) binds with high affinity to activated endothelial cells (e.g. in growing tumours, see Fig. 1B). Physiological approach for detection of live endothelial cells (e.g. cultured in vitro) is their uptake acetylated-LDL labelled with fluorochrome DiI (DiI-Ac-LDL uptake) although the same component can be also taken up by other cells, e.g. synovialocytes.
 |
 |
 |
| Fig. 1 A. Immunoperoxidase detection of von Willebrand factor in the spleen. Reaction reveals the lining of venous sinusoids inside of the red pulp. Counterstained with haematoxylin.
Author of the microfotograph: Jaroslav Mokrý. |
Fig. 1 B. UEA-1 lectin histochemistry. Reaction shows endothelial cells of blood vessels; to increase the intesity of the staining the reaction was amplified with biotinylated thyramin.
Author of the microfotograph: Jaroslav Mokrý. |
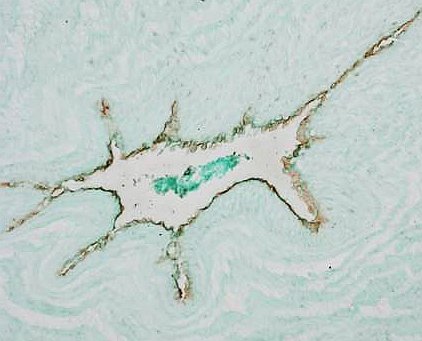
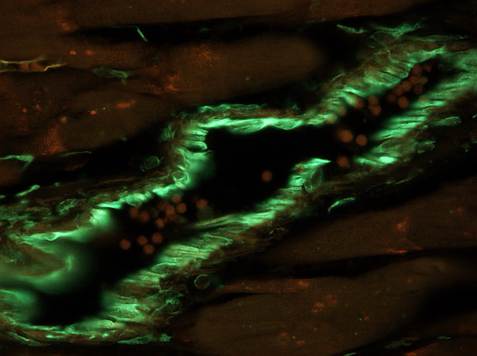
Fig. 1 C. Immunofluorescent detection of vimentin. Endothelial cells like other mesenchymal cells contain vimentin (green); detection identified the endothelium of muscular artery.
Author of the microfotograph: Jaroslav Mokrý. |
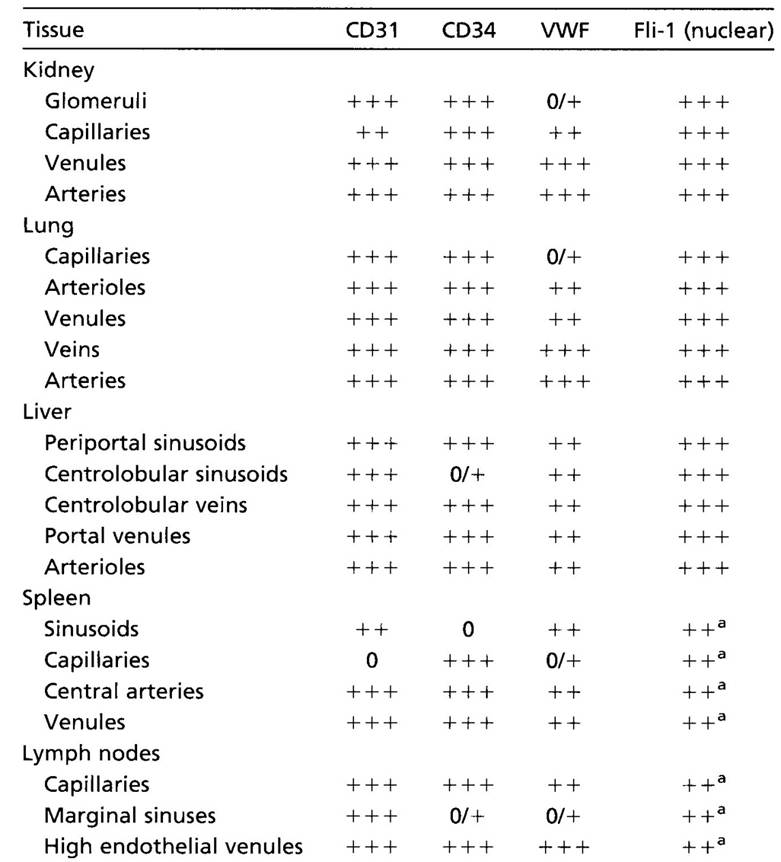
Endothelial cells are very heterogenic population. Different types of capillaries and vessels are lined with endothelium that expresses different markers: Ephrin-B3 is expressed in endothelial cells of arteries; Ephrin-B4, COUP-TFII in endothelium of veins; endothelium of lymphatic vessels express Lyve-1, Prox-1, VEGFR-3, podoplanin, and D2-40. Endothelial cells of tumours resemble activated cells and bear other markers, e.g. Tie-1,-2, VEGFR-2 and some integrins. Endothelium of haematoencephalic barrier is completely distinct by different properties and markers.
 |
||
| Tab. 1. Heterogeneity of endothelial cells. Expression of selected markers depends on the tissue and type of the blood vessel. |
2. ENDOTHELIAL CELL ORIGIN
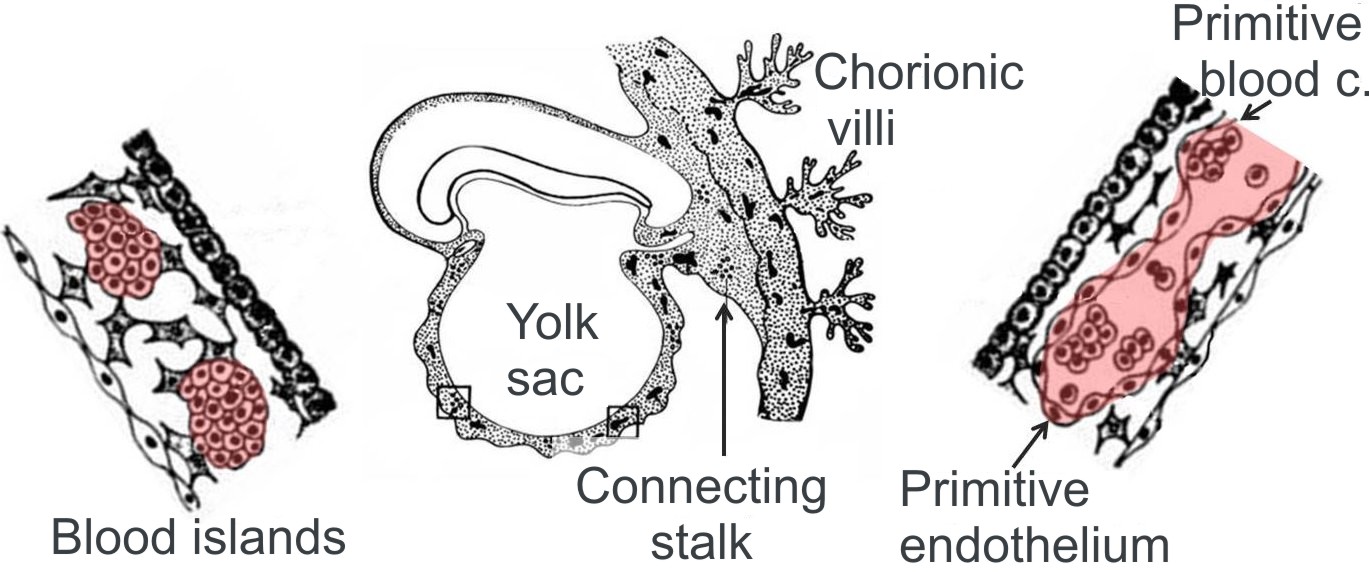
During embryonic development, endothelial cells develop from the mesoderm where they form blood islands (Fig. 2). A hypothetic cell, which gives rise to the island, is called haemangioblast. This stem cell produces angioblasts, precursors of endothelial cells (that differentiate into endothelial progenitor cells) as well as haemopoetic stem cells responsible for haemopoiesis. The study performed by Zovein et al. (2008) indicates that a definitive haemopoiesis arises from endothelial cells of blood islands - VE-cadherin+ endothelial cells migrate in the foetal liver and later in the bone marrow where they initiate haemopoiesis. Another developmental source of endothelial cells can be represented by neuroectodermal cells from the neural crest (i.e. neural crest stem cells), which participate in formation of cardiovascular structures like semilunar valves, heart output/outflow, wall of the aorta and arteries of the aortic arches, vascular smooth muscle cells and pericytes. Pericytes of the blood vessels function as endothelial cell precursors.
 |
 |
|
| Fig. 2 A. Scheme of blood island origin. Haemopoiesis begins in blood islands - haemangioblasts (image on left) give rise to islands lined by the endothelium and containing blood cells (image on right). First blood islands appear in extraembryonc mesoderm of yolk sac, connecting stalk and chorion.
Modified from Klika et al. Embryologie 1986. |
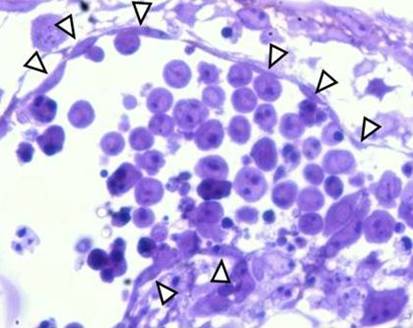
FIg. 2 B. Semithin section of blood island in the embryonic body formed by spontaneous differentiaation of ES cells. Endothelial cells (arrows) line the edge of the island while blood cells accumulate in the lumen.
Author of the microphotograph: Jaroslav Mokrý. |
Furthermore endothelial cells can also arise from other stem cells. Mesenchymal stem cells incl. bone marrow stromal stem cells participate in postnatal neovascularization. In an experiment, vascular progenitors can be derived from ES cells (Yamashita et al., 2000); similar cells have not been isolated from any other tissue so far. Endothelial cells have been also isolated from tissue-specific stem cells like dental pulp stem cells, cardiac stem cells or neural stem cells.
Ability to generate endothelial-like cells was demonstrated also in other cell types and documented, e.g. in dendritic cells, CD14+ monocytes or mast cells. Similarly to blood endothelial cells that arise from progenitor cells (endothelial progenitor cells), lymphatic endothelial cells also arise from their specific progenitor.
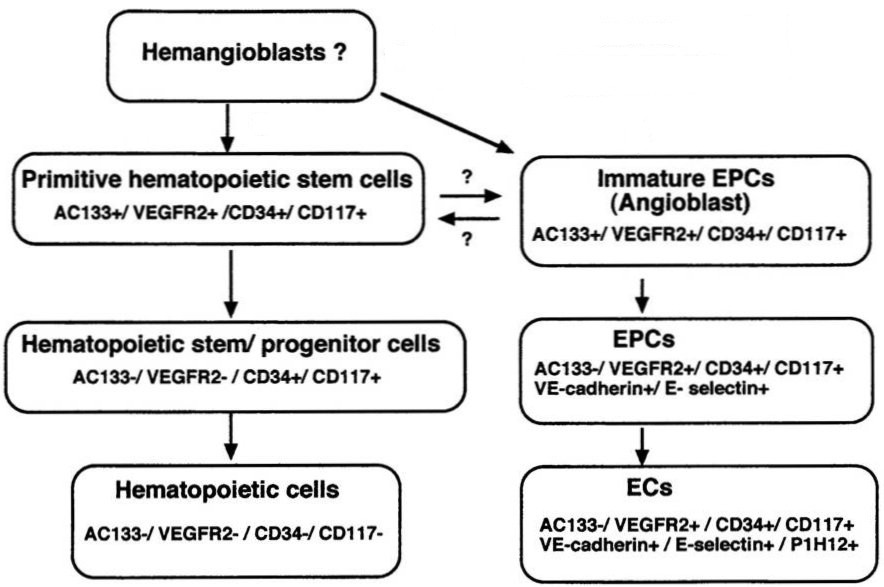 |
||
| Fig. 3. Scheme of development of endothelial cells. Haemopoetic stem cells and endothelial progenitor cells (EPCs) arise from the haemangioblast. In mice both cell types can substitute each other. With subsequent differentiation cells express distinct markers. ECs, endothelila cells. Modified from Masuda and Asahara, Cardiovasc. Res. 2003, 58, 390-398. |
3. ISOLATION OF ENDOTHELIAL PROGENITOR CELLS
| Mononuclear cells from the blood are centrifuged on Ficoll™ and seeded for 24 hrs in wells coated with fibronectin and containing culture medium EndoCult® Liquid Medium; population of adhering cell is removed. Non-adhering cells are yielded after 2 days and seeded in culture dishes coated with fibronectin. After 3 following days, the first colonies appear (see Fig. 4). Founder cells of the colonies, endothelial progenitor cells, are described as CFU-EC (colony-forming unit-endothelial cells). From 1 ml of blood up to 35,000 endothelial progenitor cells can be isolated. | ||||
 |
 |
 |
||
| Fig. 4. Isolation of endothelial progenitor cells. Shortly after cell seeding (Fig. In left – 2 days) cell culture contains single cells only. Four days after seeding (middle image) CFU-EC proliferate and give rise to the first colonies that increase their size in the following dates (Fig. In right – 5 days). Photographs were taken from the brochure on Endocult Liquid Medium. | ||||
| Endothelial progenitors express specific markers and they also are able to form tubular structures (similar to blood capillaries) in 3D gels (formed e.g. by collagen type I) (Fig. 5). | ||||
 |
 |
 |
||
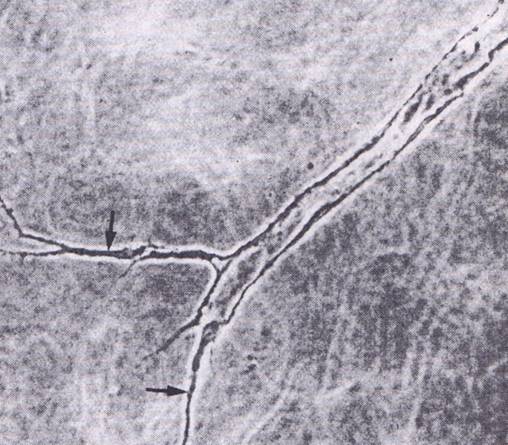
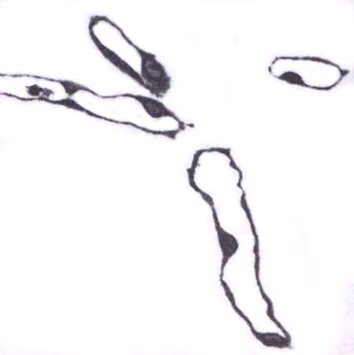
| Fig. 5. In vitro differentiation of endothelial cells. Phase contrast of tubular structures formed by endothelial cells grown in 3D gels (Fig. on left). A central part has a clear lumen whereas edge parts (arrows – middle image) have not been luminized so far. In a semithin section, the endothelium looks like a simple squamous epithelium lining an empty lumen (Fig. on right). | ||||
4. VASCULARIZATION
Process of blood vessels formation (i.e., vascularization) inside of tissues comprises different mechanism. In adulthood these processes can occur through different developmental processes.
 The term vasculogenesis covers formation of blood vessels in situ or remodelling of pre-existing blood vessels. This process leads to differentiation of angioblasts or endothelial progenitor cells into endothelial cells.
The term vasculogenesis covers formation of blood vessels in situ or remodelling of pre-existing blood vessels. This process leads to differentiation of angioblasts or endothelial progenitor cells into endothelial cells.
Angiogenesis means de novo capillary formation by endothelial cells of pre-existing capillaries or post-capillary venules. Basic mechanisms participating in angiogenesis include endothelial sprouting (Fig. 6 on left), migration, proliferation, proteolysis of extracellular matrix, tube formation followed by vascular wall remodelling.
Fig. 6. Schematic drawing of angiogenesis. Image on left explains the effect of VEGF (vascular endothelial factor) released by growing tumour in activation of adjacent endothelial cells. Changes in phenotype of endothelial cells occur through expression of different molecules participating in angiogenesis.
Arteriogenesis comprises de novo formation of arteries and growth of collateral arteries. Main mechanisms responsible for arteriogenesis include vascular wall cell proliferation, migration and wall remodelling. Fluid shear stress acts as an inductor that triggers arteriogenesis by activating the endothelium, which results in expression of adhesive molecule MCP-1, which enables mononuclear cells (monocytes and lymphocytes) to invade and initiate the inflammation. These changes are followed by the proliferative phase when mitotic division occur in endothelial and smooth muscle cells. This is followed by remodelling phase that transforms a thin-walled blood vessel to an artery with the help of proteolysis of extracellular matrix, destruction of inner elastic membrane, cell migration and formation of the neointima. New space for the growth of a new artery is obtained by lysis of the neighbouring tissue and cell death. Remodelation is finished by synthesis of new extracellular matrix components.
5. INTERMEDIATE FILAMENT NESTIN
Nestin is a 220 kDa protein (Fig. 7) found in the cytoskeleton of certain cell types; it belongs to intermediate filaments class VI. This protein appears transiently in cell development as it modifies properties of the cytoskeleton and makes it more flexibille. High levels are usually observed in dividing cells. In the course of cell differentiation nestin is changed for another and definitive type of intermediate filament: in neurons nestin is replaced with α-internexin, peripherin or neurofilaments; in astroglia, nestin is replaced with vimentin and GFAP; in muscle cells nestin is replaced with vimentin and later with desmin.
 |
 |
|
 |
||
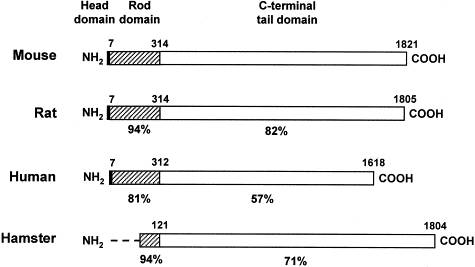
| Fig. 7. Schematic structure of nestin. Central part of the molecule is conserved (amino acids 7 to 314); helical domain participates in coiling of the intermediate filament. C-terminal part comprises charged amino acids, glutamate residues and motif of 11 repetitive amino acids. Lower image demonstrates interspecies differences in nestin structure. | Fig. 8. Immunoperoxidase detection of nestin in blood vessels of the human corpus luteum. Hormonal stimulation triggers a rapid growth of this endocrine tissue. Its nutrition occurs by newly formed blood vessels, which are lined with ednothelial cells strongly expressing nestin; the section is coverstained with light green. Author of microphotography: Jaroslav Mokrý. |
|
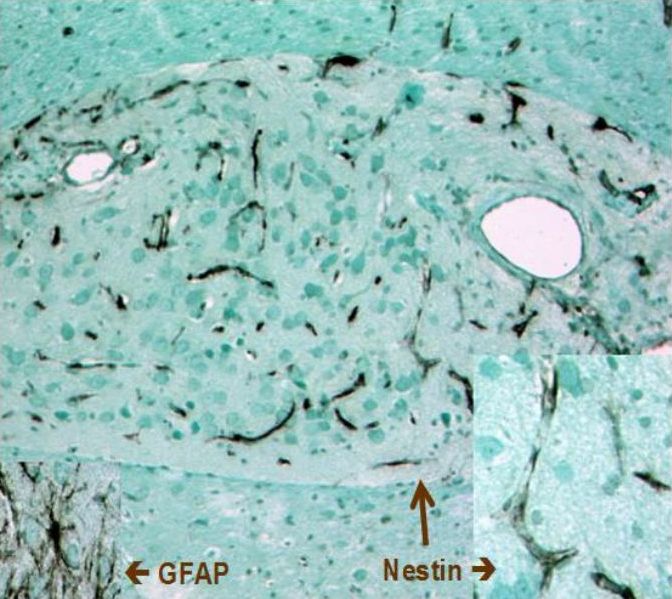
Nestin was originally identified in neuroepithelial stem cells (Lendahl et al., 1990); its name was derived from acronymum NEuroepithelial STem Cell ProteIN. It is very often considered as a marker of neural stem cells, which is not completely right as it is expressed also in other cell types. Nestin is expressed in other neural cells including radial glia, neural precursor cells, oligodendrocyte precursore, premitotic neuroblasts, reactive astrocytes and Schwann cells; of brain tumours nestin was found in gliomas. Nestin appears in developing muscles, in cells of presomitic mesoderm, myotome and also in developing cardiac muscle. Other nestin-positive cells include mesonefric mesenchyma, podocytes, Sertoli cells, odontoblasts or Ito cells. Our group was the first to identify nestin expression in endothelial cells (Mokrý and Němeček, 1998; 1999 - Figs. 8 and 9). Fig. 9. Nestin expression in blood capillaries of neural graft. Angiogenesis of blood capillaries provided nutrition to the transplanted tissue. In 3 weeks after transplantation neural cells differentiated (and therefore they were not containing nestin) whereas endothelium of newly formed blood capillaries express high levels of nestin. Immunoperoxidase reaction of nestin; counterstained with light green.Author of microphotograph: Jaroslav Mokrý. |
 |
6. THERAPEUTIC IMPLICATIONS
Endothelial progenitor cells participate in processes linked with tissue regeneration and growth especially in situations associated with changes in distribution of EPCs. Circulating endothelial progenitor cells (cEPCs, i.e. those occurring in the peripheral blood) (circulating EPCs) home actively to sites of neovascularization; cEPCs are recruited to tumours and participate in tumour angiogenesis. Recent studies provided evidence that cEPCs are mobilized in response to tissue ischemia, e.g. in acute myocardial infarction, coronary artery bypass grafting, burns etc.
A decrease in circulating endothelial progenitor cells was observed in patients with coronary artery disease (impaired vascularization) and also in old, diabetic and hypercholesterolemic patients. A drop in cEPCs reflects endothelial dysfunction.
Above mentioned examples document active participation of endothelial progenitor cells in process of endothelial lining renewal in blood vessels. For that reason EPCs are promising candidates for cell therapy to support tissue vascularization. In experiments aimed at utilization of EPCs in therapy of lower limb ischemia transplantation of these cells improved limb perfusion whereas administration of endothelial (i.e. differentiated) cells failed. Infusion of endothelial progenitor cells in myocardial infarction preserved left ventricular function, enhanced neovascularization and inhibited myocardial fibrosis. Bone marrow-derived EPCs control the angiogenic switch in mouse lung metastasis.
Another possible implication can be seen in anti-angiogenic therapy focused on eradication of endothelial progenitor cells under conditions where angiogenesis should be suppressed (e.g. to suppress nourishment of a growing tumour).
7. NESTIN EXPRESSION IN BLOOD VESSELS
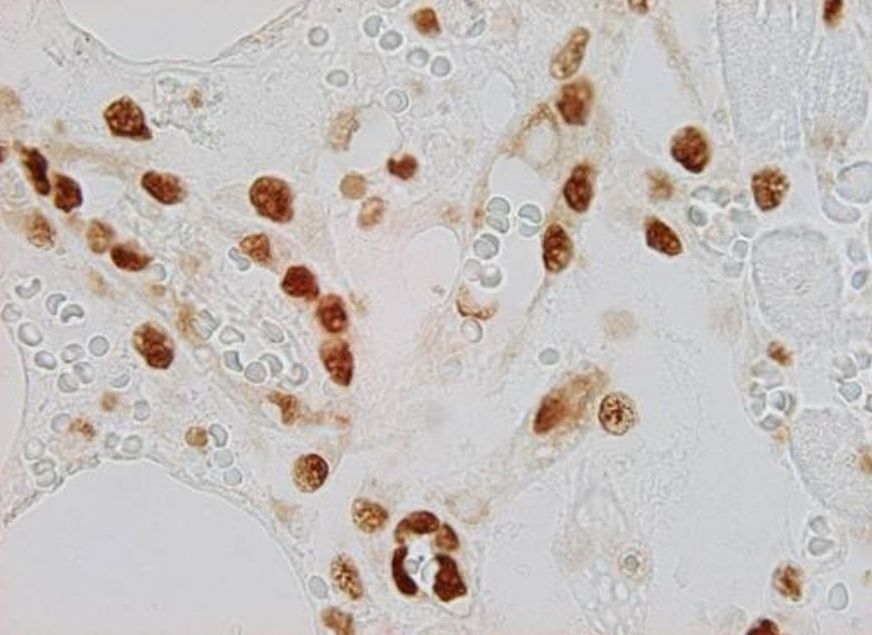
Nestin is expressed in newly formed endothelial cells of blood capillaries, i.e. endothelial cells expressing nestin also co-express proliferative markers (e.g., PCNA). For that reason nestin can be identified in blood vessels of developing organs not only in intraembryonic but also in extraembryonic tissues including placenta (Mokrý and Němeček, 1998 – see Fig. 10). In adult tissues which have fully developed blood vessels nestin can be detected in transplants, e.g. neural ones, which are vascularized by ingrowing blood vessels (Mokrý and Němeček, 1999). Similarly nestin can be identified in vascular endothelium of growing tumours and also in physiologically growing tissues like in the corpus luteum or endometrium. Nestin-positive vessels also appear in regeneration. In patients after myocardial infarction nestin-immunoreactive blood vessels are growing toward the ischaemic tissue (Mokrý, J. et al. 2004 - Fig. 11).
 |
 |
|
| Fig. 10. Immunohistochemical detection of nestin in blood vessels of placenta. Newly formed blood vessels in chorionic villi of human placenta (left image) are lined with nestin-positive endothelium (brown) while erythrocytes and section background are not stained. In rat placenta chorionic villi contain small and large blood vessels (right image); lumen of large blood vessels is indicated by asterisk. Immunoperoxidase reaction; counterstained with light green. Author of microphotograph: Jaroslav Mokrý. |
||
 |
 |
|
| Fig. 11. Immunohistochemical detection of nestin in v regenerating blood vessels after myocardial infarction. Immunofluorescent detection of nestin (left image - red) in the cytoplasm of endothelium of newly formed blood vessels. Nuclei of nestin-positive endothelial cells expressing PCNA (right image - brown) provide evidence of newly formed cells. Author of microphotograph: Jaroslav Mokrý. |
||